CeraOss® HYA: the innovative 2-in-1 combination of bovine bone and hyaluronic acid

CeraOss HYA is a bone substitute that combines the properties of natural bovine bone (CeraOss) with the advantages of manipulation and tissue regeneration of hyaluronic acid. While bone particles provide an osteoconductive scaffold and ensure permanent volume stability at the augmentation site, hyaluronic acid forms a malleable mass that improves graft handling and facilitates its application in surgery. CeraOss HYA therefore offers an ideal synergy between ease of use and long-term volume stability.
Product features*
- Simplified handling
After hydration with saline or blood, CeraOss HYA is mixed in the original blister to yield a graft with a sticky consistency commonly called the sticky bone. The advantageous malleability of the sticky bone provides comfort in application and speeds up surgery.1, 2
- Structure comparable to human bone
Bovine bone granules have a porosity of ≈65-80% and an interconnected network of macropores (facilitate the penetration of osteogenic cells and blood vessels) and micropores (stimulate the absorption of liquids by capillarity). In addition, the rough surface of CeraOss HYA promotes the adhesion of osteoblasts and signaling molecules and thus contributes to the integration of bone particles.3, 4
- Stimulation of angiogenesis
The chorioallantoic membrane test revealed that CeraOss HYA stimulates the formation of blood vessel in vivo, at a higher density than an identical material with no hyaluronic acid added.5
- Increased cell activity
The viability, proliferation, and migratory activity of human osteoblasts were improved when cells were cultured in vitro with CeraOss HYA, compared to those cultured with a bone substitute without added hyaluronic acid.6
- Promotion of bone regeneration
A recent study showed that hyaluronic acid promotes the formation of mineralized and non-mineralized bone matrix.8
- Long-term volume stability
The bovine bone granules that make up CeraOss HYA are reabsorbed very slowly, and therefore offer long-lasting structural support. This volume stability is particularly important in the aesthetic zone or when aiming to preserve the contour of the alveolar ridge.9, 10 In addition, mixing CeraOss HYA with autologous or allogeneic bone results in a graft that combines volume stability with accelerated bone regeneration capacity.11
- Safe
Processing the bovine bone at high temperature (>1200 °C) removes any potentially infectious agents such as bacteria, viruses and prions.12
- Biocompatible and non-immunogenic
In vivo analysis of biocompatibility demonstrated that the inflammatory and immune response to CeraOss HYA was comparable to that of similar bovine bone without added hyaluronic acid.13
- Naturally absorbable biopolymer
Histological monitoring has shown that hyaluronic acid is reabsorbed by enzymatic degradation after a two-week postoperative period.13
- Effective for the treatment of peri-implantitis
A randomized clinical trial revealed significant vertical bone gains in peri-implant bone defects that were treated with CeraOss HYA. In addition, an improvement in implant stability was observed at 3 and 6 months postoperative.14
*Studies were conducted using bone substitute materials that are identical to CeraOss and CeraOss HYA.
CeraOss HYA – Benefits for regeneration
- CeraOss HYA stimulates the formation of blood vessels in vivo5 and enhances the biological activity of osteoblasts in vitro.6, 7
- Improves bone regeneration.14
- Increases implant stability.14
Randomized, Controlled Clinical Study of Reconstructive Surgery for Peri-Implantitis
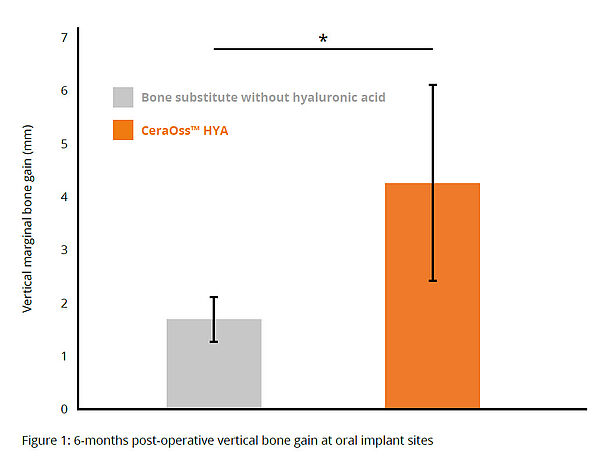
Compared to patients treated with CeraOss, those treated with CeraOss HYA had greater vertical bone gain, statistically significant in mesial, distal and buccal implants, at 6 months postoperative (*p < 0.05) (Fig. 1).14
Exceptional fluid retention
Sodium hyaluronate is the conjugate base of hyaluronic acid, an unsulfated anionic glycosaminoglycan, widely distributed in connective and epithelial tissues. Hyaluronic acid is one of the most hygroscopic molecules that can absorb a thousand times its weight in water. After hydration, hydrogen bonds form between the water molecules and the carboxyl and N-acetyl groups of hyaluronic acid. These interactions result in a three-dimensional mass of malleable consistency that captures the bovine bone granules and allows easy application of the graft. Hyaluronic acid serves therefore as a carrier for bovine bone granules.
Hyaluronic Acid structure
Hyaluronic acid is a biopolymer composed of D-glucuronic acid and N-acetyl-D-glucosamine units. The molecular weight is determined by the degree of polymerization (n = the number of repetitive units that make up the polymer chain). Hyaluronic acid with high molecular weight possesses extended degradation times and demonstrated anti-inflammatory effect.15
Bacteriostatic effect
The use of hyaluronic acid in the form of a membrane, gel, and sponge has been shown to have bacteriostatic effects on surgical wounds. This reduces the risk of postoperative infections and increases the predictability of defect regeneration.16
Hyaluronic acid in dentistry
Hyaluronic acid is an essential component of the periodontal ligament matrix. It influences cell adhesion, migration, and differentiation by engaging with binding proteins and cell surface receptors. The benefits of hyaluronic acid in the healing process of periodontal wounds, including inflammation, granulation tissue formation, and epithelial formation, were described in the literature.17–22 Hyaluronic acid has also been shown to induce early trabecular bone deposition in dental compartments and stimulate the expression of osteogenic proteins such as bone morphogenetic protein type 2 (BMP-2) and osteopontin. 23
1 Cerabone® plus usability test.
2 78.5% of users reported easier or much easier application compared to particulate material without hyaluronic acid; Data on file: Customer survey among 156 clinicians.
3 Tadic et al. Comparison of different methods for the preparation of porous bone substitution materials and structural investigations by synchrotron μ-computer tomography. Mat.-wiss. u. Werkstofftech. 2004, 35, No. 4.
4 Seidel and Dingeldein 2004. cerabone® – Bovine Based Spongiosa Ceramic Seidel et al. Mat.-wiss. u. Werkstofftech. 35:208–212.
5 Kyyak et al. Hyaluronic Acid with Bone Substitutes Enhance Angiogenesis In Vivo. Materials (Basel) 2022. 15(11):3839.
6 Kyyak et al. The Influence of Hyaluronic Acid Biofunctionalization of a Bovine Bone Substitute on Osteoblast Activity In Vitro. Materials (Basel). 2021. 14(11):2885.
7 Qasim SSB, Trajkovski B, Zafiropoulos GG. The response of human osteoblasts on bovine xenografts with and without hyaluronate used in bone augmentation. J Biomater Sci Polym Ed. 2024 Apr;35(6):880- 897. doi: 10.1080/09205063.2024.2311454. Epub 2024 Feb 12. PMID: 38346177.
8 Zhao, N., Wang, X., Qin, L., Zhai, M., Yuan, J., Chen, J., & Li, D. (2016). Effect of hyaluronic acid in bone formation and its applications in dentistry. Journal of biomedical materials research Part A, 104(6), 1560-1569.
9 Tawil et al. 2018. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int J Oral Maxillofac Implants.33(5):1089-1096.
10 Riachi et al. 2012. Influence of material properties on rate of resorption of two bone graft materials after sinus lift using radiographic assessment. Int J Dent. 2012:737262.
11 Kloss et al. First Clinical Case Report of a Xenograft-Allograft Combination for Alveolar Ridge Augmentation Using a Bovine Bone Substitute Material with Hyaluronate (Cerabone® Plus) Combined with Allogeneic Bone Granules (Maxgraft®). J Clin Med. 2023. 12(19):6214.
12 Brown et al. New studies on the heat resistance of hamster-adapted scrapie agent: threshold survival after ashing at 600 degrees C suggests an inorganic template of replication, PNAS 2000. 97(7): 3418–3421.
13 Pröhl A et al. In Vivo Analysis of the Biocompatibility and Bone Healing Capacity of a Novel Bone Grafting Material Combined with Hyaluronic Acid. Int J Mol Sci. 2021. 22(9):48
14 Rakašević et al. Reconstructive Peri-Implantitis Therapy by Using Bovine Bone Substitute with or without Hyaluronic Acid: A Randomized Clinical Controlled Pilot Study. J Funct Biomater. 2023 Mar 8;14(3):149.
15 Rayahin, J. E., Buhrman, J. S., Zhang, Y., Koh, T. J., & Gemeinhart, R. A. (2015). High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS biomaterials science & engineering, 1(7), 481-493.
16 Pirnazar P. et al. ’Bacteriostatic effects of hyaluronic acid. Journal of Periodontology 1999. 70:370-374.
17 Håkansson et al. Regulation of granulocyte function by hyaluronic acid. In vitro and in vivo effects on phagocytosis, locomotion, and metabolism. J Clin Invest. 198066:298–305.
18 Wisniewski HG, Vilcek J. TSG-6: An IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997. 8:143-56.
19 Larjava et al. Characterization of one phenotype of human periodontal granulation-tissue fibroblasts. J Dent Res. 1989. 68:20-25.
20 Bartold PM, Page RC. The effect of chronic inflammation on gingival connective tissue proteoglycans and hyaluronic acid. J Oral Pathol. 1986. 15:367-74.
21 Bertolami CN, Messadi DV. The role of proteoglycans in hard and soft tissue repair. Crit Rev Oral Biol Med. 1994. 5:311-37.
22 Ruggiero et al. Hyaluronidase activity of rabbit skin wound granulation tissue fibroblasts. J Dent Res. 1987. 66:1283-7.
23 Mendes et al. Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch Oral Biol. 2008. 53:1155-62.
CeraOss® – bovine bone graft substitute

CeraOss is a 100 % pure bone mineral of bovine origin manufactured by a unique 1200 °C production process. Its three-dimensional porous network enables a fast penetration and adsorption of blood and serum proteins and serves as a depot for proteins and growth factors. The unique processing ensures maximum safety and leads to an exceptionally high purity of CeraOss, providing ultimate volume stability of the augmentation site.1-3
Ideal for following indications
- Alveolar ridge augmentation/reconstruction
- Filling of bone defects (including after root resection,apicoectomy or cystectomy)
- Filling of extraction alveoli to support alveolar ridge preservation
- Sinus lift procedure
- Filling of periodontal bone defects
- Filling of extraction sockets as part of immediate implantations
- Filling of peri-implant bone defects